Background:In patients with Waldenström macroglobulinemia (WM), continuous once-daily ibrutinib-based therapy has been associated with long-term progression-free survival. In addition, patients who continued ibrutinib-based treatment had better survival outcomes than those who discontinued treatment within the first few years. Evidence based on a single-center retrospective study suggests that dose reduction (DR) can be used to effectively manage ibrutinib-related adverse events (AEs) and avoid early treatment discontinuation. However, real-world data on outcomes in patients with WM after ibrutinib DR are limited. In this real-world study, we analyzed short-term (1-year follow-up) dosing patterns and time to treatment discontinuation (TTD) in patients with WM, with and without DR, who were receiving first-line (1L) ibrutinib.
Methods: Medicare Fee-for-Service closed-claim medical or pharmacy records were used to identify patients diagnosed with WM who initiated 1L single-agent ibrutinib (420 mg/day) from January 2014 to December 2020. Observed patients were enrolled in Medicare ≥12 months before and after ibrutinib initiation and had post-initiation AEs (both prevalent and incident AEs with DR recommendation from the US Prescribing Information), as identified via ICD-9 or ICD-10 codes. Ibrutinib DR was defined as a reduction of the starting dose following the first AE within the 1-year follow-up period. Patients without a DR were defined as those who had received ibrutinib 420 mg per day during the 1-year follow-up period. Patients with a DR in the absence of AEs and patients with a DR prior to any AE were excluded from the analysis. Demographics, clinical characteristics, time to first AE, and TTD were analyzed among cohorts with and without DR. TTD was defined and evaluated as the time from first observed AE after ibrutinib initiation to the earliest occurrence of (1) a gap of >120 days between the end of 1L therapy and the follow-up period, (2) initiation of a next line of therapy, or (3) death. TTD was analyzed using Kaplan-Meier methodology, log rank test, and a Cox proportional hazards model adjusted for baseline and time-varying covariates.
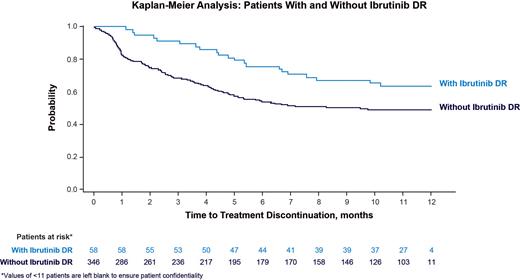
Results: We identified 404 patients (DR, n = 58 [14%]; no DR, 346 [86%]). Overall, 249 patients (62%) were men, 267 patients (66%) had hypertension, 55 patients (14%) had atrial fibrillation, and 114 patients (28%) had other cardiovascular conditions at baseline. The mean ± SD age at baseline was 77 ± 8 years. In both cohorts, the median Charlson Comorbidity Index was 4.0. Among the 404 patients, mean ± SD time from ibrutinib initiation to first AE was 28 ± 44 days. Mean ± SD time between first AE and DR was 137 ± 102 days. Mean TTD was 8.3 months (DR) and 6.2 (no DR) ( P = 0.018; Figure). At 6 and 12 months of follow-up, fewer patients with DR discontinued treatment compared with patients without DR (6 months: 24% vs 46%, P = 0.002; 12 months: 36% vs 50%, P = 0.064). After controlling for baseline and time-varying demographic and clinical characteristics, patients with DR were not significantly more likely to discontinue treatment compared with patients without DR (hazard ratio [95% CI], DR vs no DR: 1.24 [0.86-1.78]).
Conclusion:These short-term (1-year follow-up) findings suggest TTD was not impacted by DR of ibrutinib following AEs. Thus, ibrutinib DR can be an effective strategy to manage AEs while maintaining clinical efficacy. Of note, causal association between AE and subsequent DR cannot be inferred from the claims database due to the cross-sectional nature of this study.
Disclosures
Sarosiek:ADC Therapeutics: Research Funding; Beigene: Honoraria, Research Funding; Cellectar: Consultancy, Research Funding. von Keudell:TG Therapeutics: Research Funding; Syndax: Research Funding; Janssen: Research Funding; Merck: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Pharmacyclics, an AbbAvie Company: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding. Paludo:Biofourmis: Research Funding; AbbVie: Consultancy; Karyopharm: Research Funding. Salkar:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Agatep:Sanofi: Consultancy; AbbVie: Consultancy; Inovalon: Current Employment. Crawford:Kite Pharma: Current Employment; Stratevi: Current Employment; AbbVie: Current Employment, Current holder of stock options in a privately-held company; Cerner: Current Employment. Pacia:AbbVie: Current Employment. Castillo:AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Cellectar: Consultancy, Research Funding; Kite: Consultancy; Mustang Bio: Consultancy; Loxo: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal